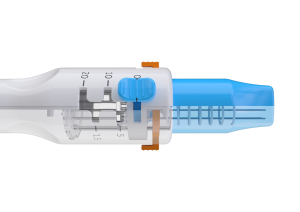
EndoVE
Electroporation with chemotherapy has been in clinical use for more than 15 years and in 2006 the European Standard Operating Procedure (ESOPE) which described the standard delivery of the procedure including chemotherapy dose.
Of great benefit, due to the greater conductivity of tumour tissue, the surrounding healthy tissue structures are not damaged in the process.
The effectiveness of electroporation in tumour ablation clinically has been reported by a growing number of clinicians in the US and Europe with excellent quality of life and tumour reduction reported for both cutaneous and intraluminal applications.
The EndoVE system has been assessed in clinical trials for patients with inoperable colorectal and oesophageal cancer. The clinical evidence to date with electroporation has demonstrated its excellent efficacy in tumour treatment even in cases where tumours were previously unresponsive to chemotherapy or radiotherapy.
Description
Features & Benefits
- Demonstrated to be safe and effective
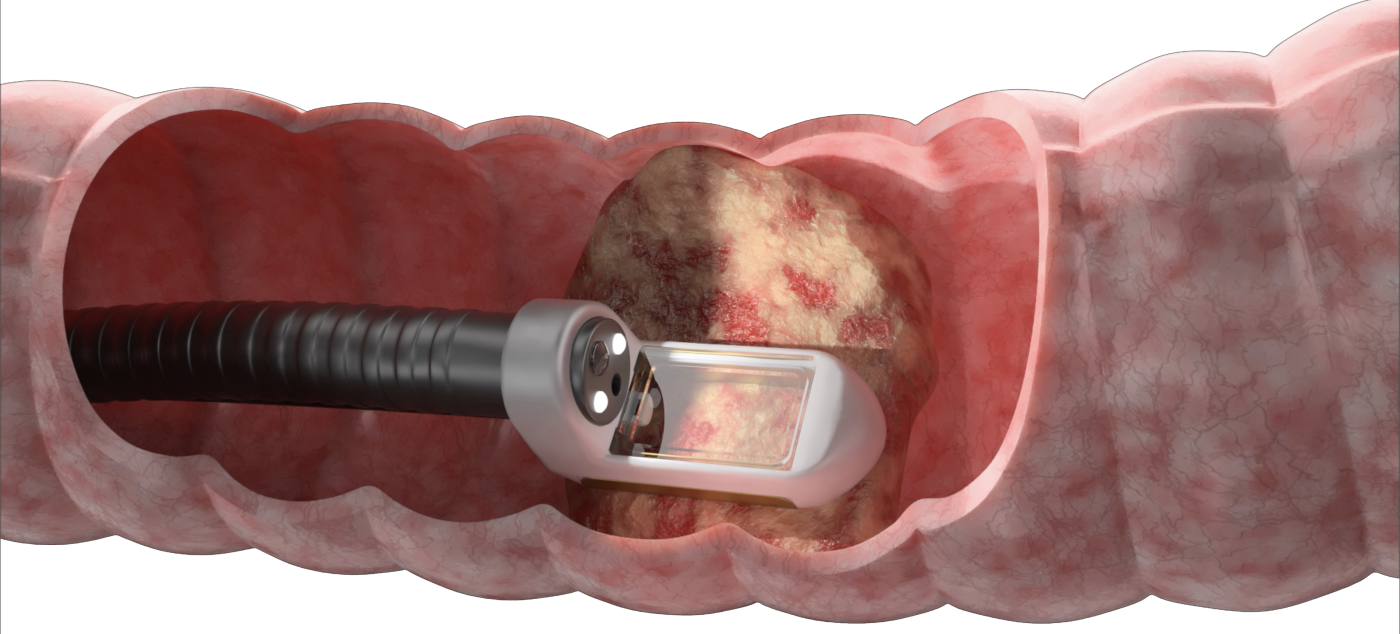
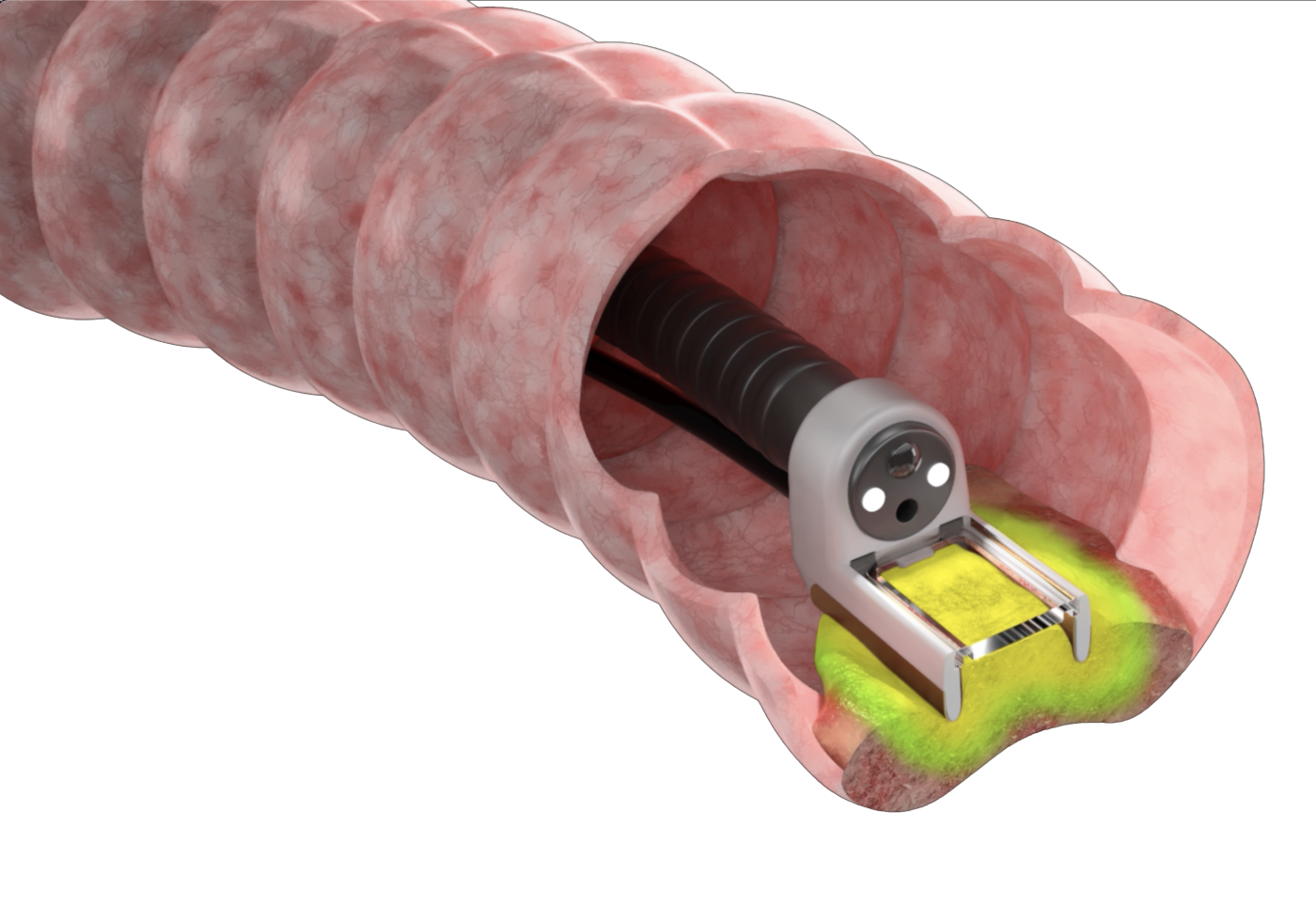
- Precision delivery of non-thermal ablation which targets tumour tissue whilst preserving surrounding healthy tissue structures
- ePORE and EndoVE are repeatable and leave standard treatment options available
- Delivered under light sedation, day case, cost effective
- Simple standard connection for endoscopes between 9mm and 10mm
- Connects to a vacuum which draws the tumour tissue into the chamber this can be viewed via the window at the head of the electrode
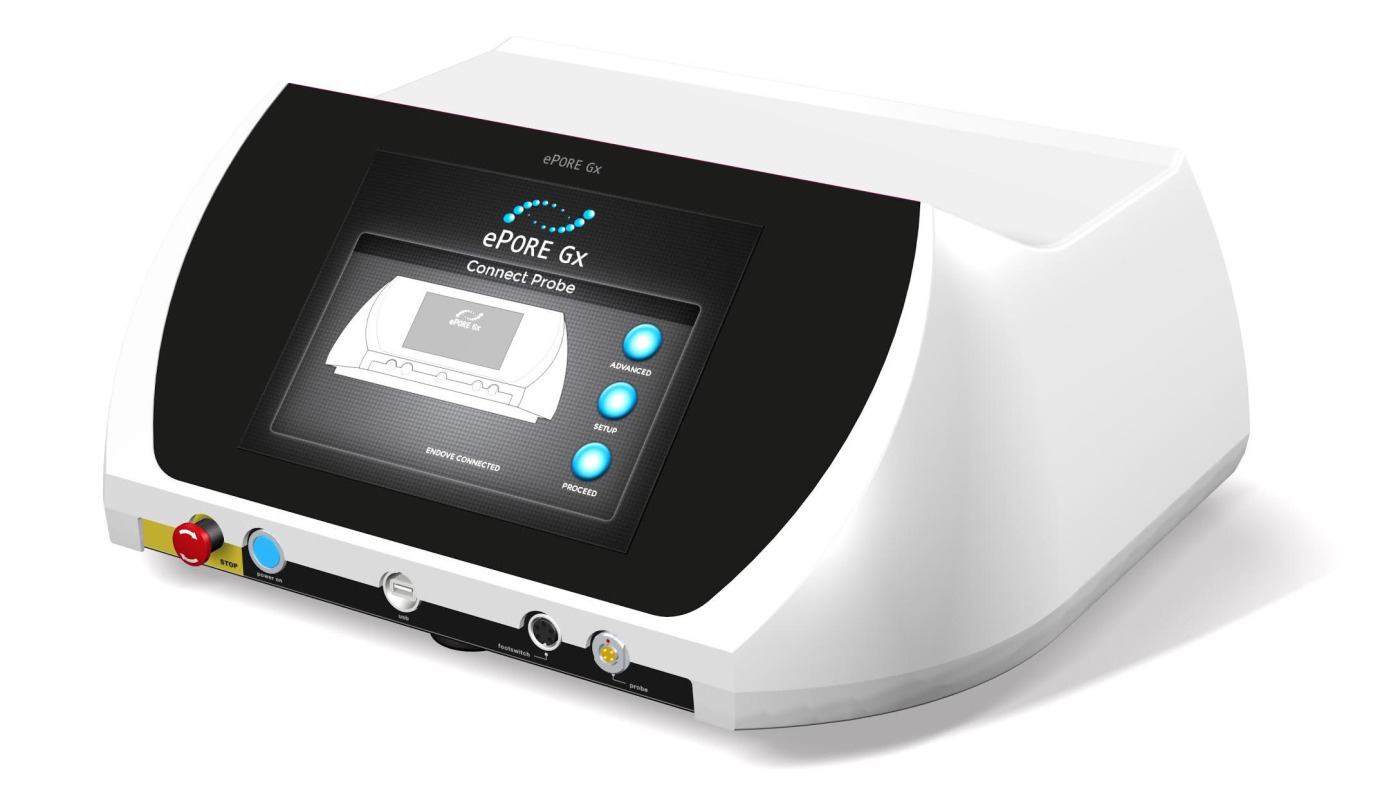
- Ease of use
|
Supplier |
Product Code |
Description |
QOM |
FAL Code |
|
Mirai Medical |
MS-18-07 |
EndoVE High Frequency Extension Lead |
1 |
|
|
Mirai Medical |
MS-19-05 |
EndoVE Low Frequency Extension Lead |
1 |
|
|
Mirai Medical |
Epore MMHT1 |
ePORe Generator MMHT1 |
1 |
|
|
Mirai Medical |
EndoVE 700209 |
EndoVE 700209 Electroporation Probe |
1 |
|