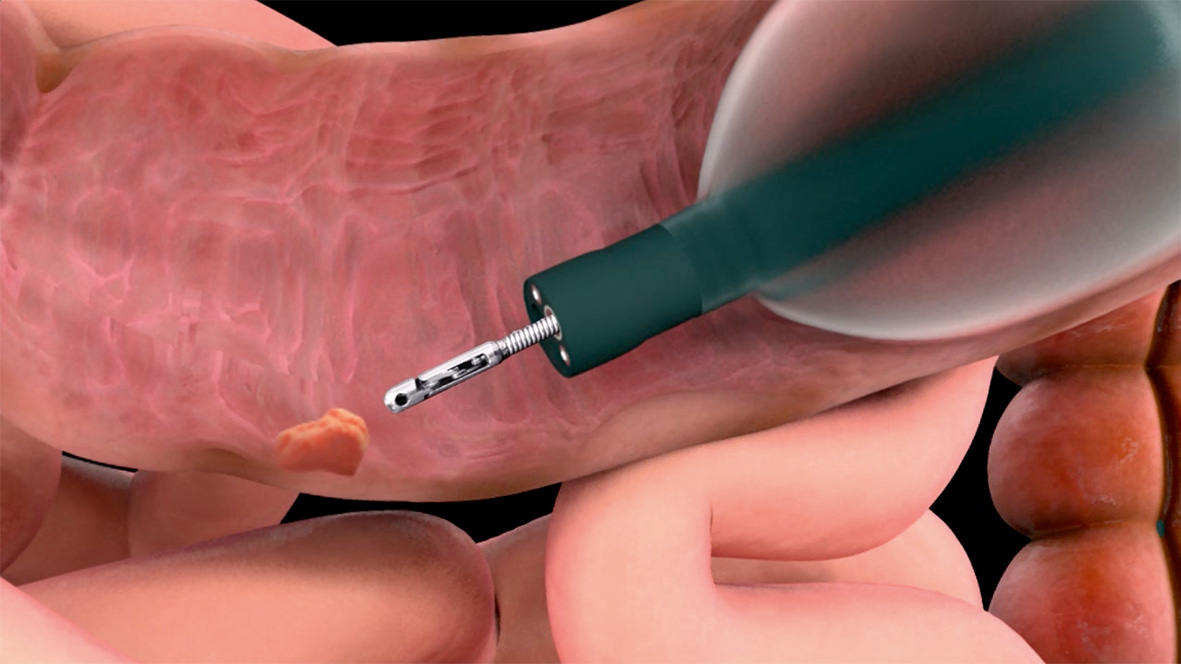
G-EYE 760 R-V/M, V/I, V/L Colonoscope à ballonnet
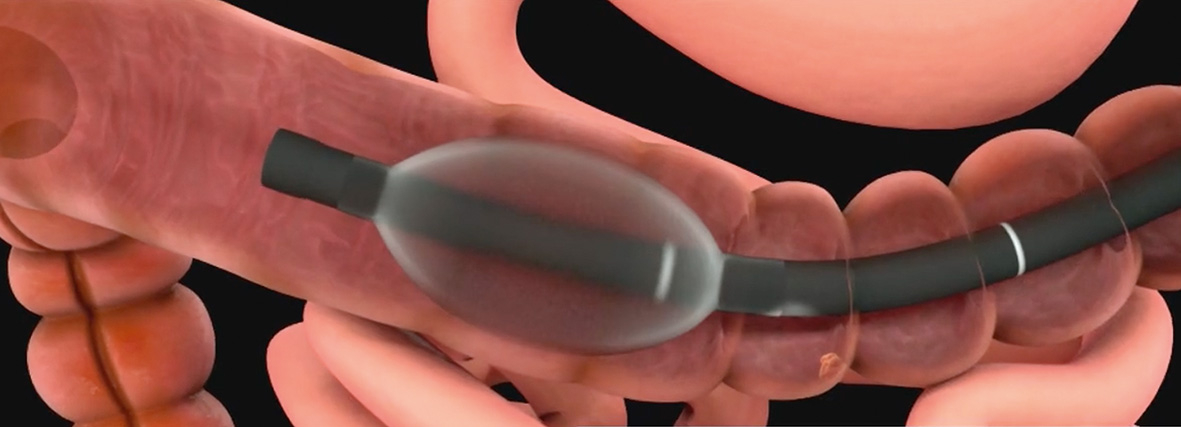
The G-EYE 760 R endoscope is equipped with a permanently integrated balloon at the bending section of the routine colonoscope. On demand, the re-usable balloon can be inflated, thereby flattening the colonic walls and improving the detection of hidden polyps. The diagnostic yield is expected to further be supplemented by increased colour contrast of the Linked Color Imaging (LCI) mode that was specifically developed to improve detection of lesions or inflammation, resulting in more accurate delineation.
Besides detection enhancement, physicians could benefit from the G-EYE system throughout the whole procedure, from assistance in delooping during intubation, via Controlled Withdrawal™ that reduces bowel slippage, through to supporting therapeutic interventions e.g. EMR/ESD by stabilising and anchoring the endoscope tip.
Fujifilm EC-760 R-V/M, V/I, V/L G-EYE Colonoscope
Prof. Ralf Kieeslich about Artificial Intelligence in practice using CAD EYE and the G-EYE Endoscope. Watch the video
Specifications
G-EYE EC-760 R-V/M, V/I, V/L
|
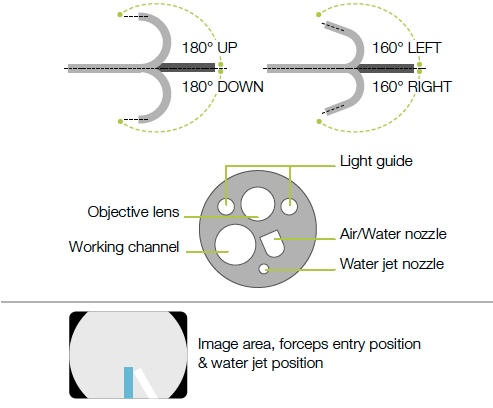
Field of view |
170° |
|
Observation range |
2 – 100 mm |
|
Bending capability* |
Up 180° / Down 180° |
|
Distal end ø |
12.0 mm |
|
Insertion tube ø |
12.0 mm |
|
Working channel ø |
3.8 mm |
|
Working length |
1330/1520/1690 mm |
|
Total length |
1650/1840/2010 mm |
|
Balloon ø |
up to 55 mm |
*when balloon is deflated
Spark2C Inflation System
|
Controlled Withdrawal™ |
Optical system centers in the lumen, minimal deflection during steady withdrawal |
|
Endoscope Model |
G-EYE EC-760 R-V/M, V/I, V/L |
|
Compatible Processor / |
ELUXEO™ VP / BL-7000 |
| Inflation System | 3 intermediate pressure levels 1 anchoring pressure level |
| Control and Foot Panel | for simple operation |
| Hand-held Leak Tester | for additional automatic leak testing prior to AER |
| Dimensions | 280 x 195 x 90 mm |
| Weight | 1.9 kg |
SPARK2C Inflation System
Inflation and deflation of the balloon can easily be controlled by the hand-held control or foot panel. Three intermediate pressure levels allow for Controlled Withdrawal™ whereas the additional anchoring pressure level can be used for anchoring during treatments. Permanent leak testing ensures full endoscope integrity throughout the procedure. Material as well as detergent compatibility was certified with all established AER and detergent manufacturers.
Documents